
The Food Science of Hard-Boiled Eggs – Part I: Boiling Water
There are three parts to the science of hard-boiled eggs: the physical chemistry of boiling water, the biochemistry of eggs, and the science of cooking eggs. We will look at them in that order over the next three Sundays. Today in part I, we will look at the science of boiling water. Have you been adding salt to the water you use to boil your eggs? I’ll show you why you probably may want to stop wasting your salt…
THE PHYSICAL CHEMISTRY OF BOILING WATER
It’s All about Vapor Pressure
If you can understand what vapor pressure is, then the science of boiling liquids will never confuse you again. Not only will you understand boiling eggs, you’ll gain ultimate comprehension over cooking rice, pasta and New England boiled dinners too. Unlocking the secrets of vapor pressure is an essential for better living through chemistry. Admit it: science in the kitchen rocks!

How Vapor Pressure Controls Boiling Temperature
Imagine a pot of water on your stove top. Don’t turn on the heat and boil the imaginary water in our imaginary pot yet. For the moment, our pot of water is sitting at room temperature, at sea level, at Pismo Beach, California. After all, that’s where Bugs Bunny and Daffy Duck were heading to dig clams when they missed taking that left turn at Albuquerque. So let’s look at the conditions prevailing at our imaginary pot of water:
- Room temperature in Pismo Beach at 68 ºF, which is 20 ºC.
- Since our pot is at sea level, there’s a column of air pushing down on top of the water in the pot, exerting a pressure of 1 atmosphere (atm), which is the same as 101.325 kiloPascals (kPa) or 14.7 pounds per square inch (psi).
As you may or may not remember from high school, at least in America, these conditions are standard temperature and pressure (STP) as defined by the U.S. National Institute of Standards and Testing (NIST). There’s another version of STP where temperature is defined as 32 ºF (0 ºC) that is often used in chemistry, but we’re going to ignore that and use the 20 ºC STP.
The column of air on top of the water in the pot can be considered to be its own thing, isolated from the rest of the atmosphere. This isn’t as far fetched as it initially sounds. Even though gas molecules are always in transit, just as many molecules enter our gas column as leave it in reality, so the sum total of gas in the column is always the same. It always weighs the same and exerts the same pressure. We can treat it as if it is its own thing despite the motion of gas molecules. We can ignore the rest of the atmosphere and deal with just our air column with its set amount of gas, weight and pressure.
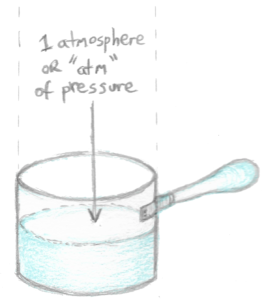
Now 1 atm of pressure is pushing down on the water in the pot. The thing is that water evaporates. The water molecules leaving the pot push back at the pressure of the air column. This is the same thing as saying that the evaporated gas molecules exert their own pressure against the pressure of the air column. This is the most basic explanation of what vapor pressure is: it’s the pressure exerted by vapor that has evaporated from a liquid. At our STP conditions of 20 ºC (68 ºF) and 1 atm, the vapor pressure of water is approximately 0.023 atm.
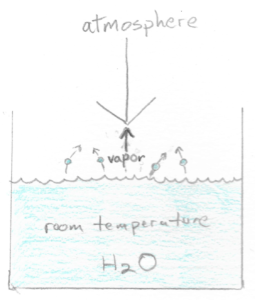
Let’s turn on the stove burner under our pot. The water molecules start to move more energetically as they heat up and this increases the number of water molecules that evaporate from the pot. As more molecules enter the vapor phase above the surface of the heating water, the greater the pressure is that they exert back at the pressure of the air column. It’s a simple matter of quantity.
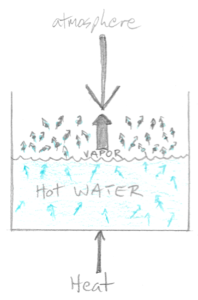
So as we add more heat to the water, the more the water molecules bounce around in the liquid. As a consequence, more and more molecules evaporate and enter the vapor phase, causing the vapor pressure to continuously increase, always pushing back at the 1 atm of pressure of the air column.
- At 50 ºC, the vapor pressure of water is 0.121 atm
- At 70 ºC, the vapor pressure of water is 0.307 atm
- At 90 ºC, the vapor pressure of water is 0.691 atm
- At 95 ºC, the vapor pressure of water is 0.834 atm
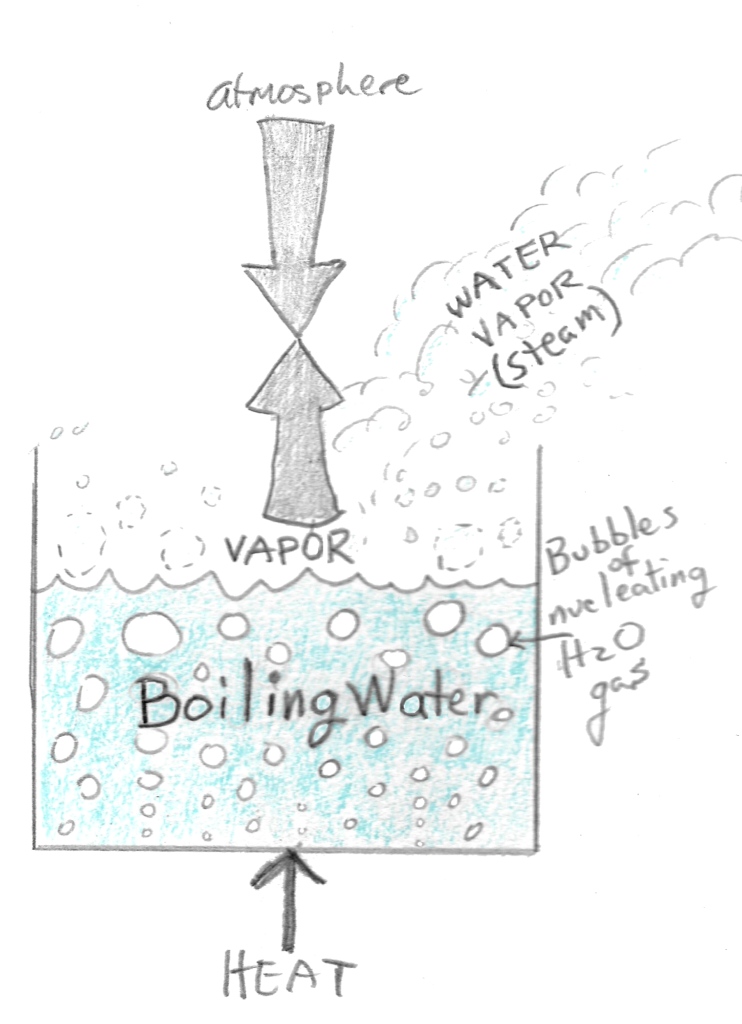
There comes a point, at 100 ºC (212 ºF), when the vapor pressure equals 1 atm. The vapor pressure now equals the pressure exerted on the water by the column of air and the water in the pot will now boil until all of it converts to gas. The temperature of the water will remain at 100 ºC until all of it has become gas. No matter how much more heat we add, the water temperature will not exceed 100 ºC. If we add a lot more heat, the water will only boil faster but it will not get any hotter. This is the behavior of water at the boiling point.
The boiling point of any liquid like water occurs when the vapor pressure equals atmospheric pressure.
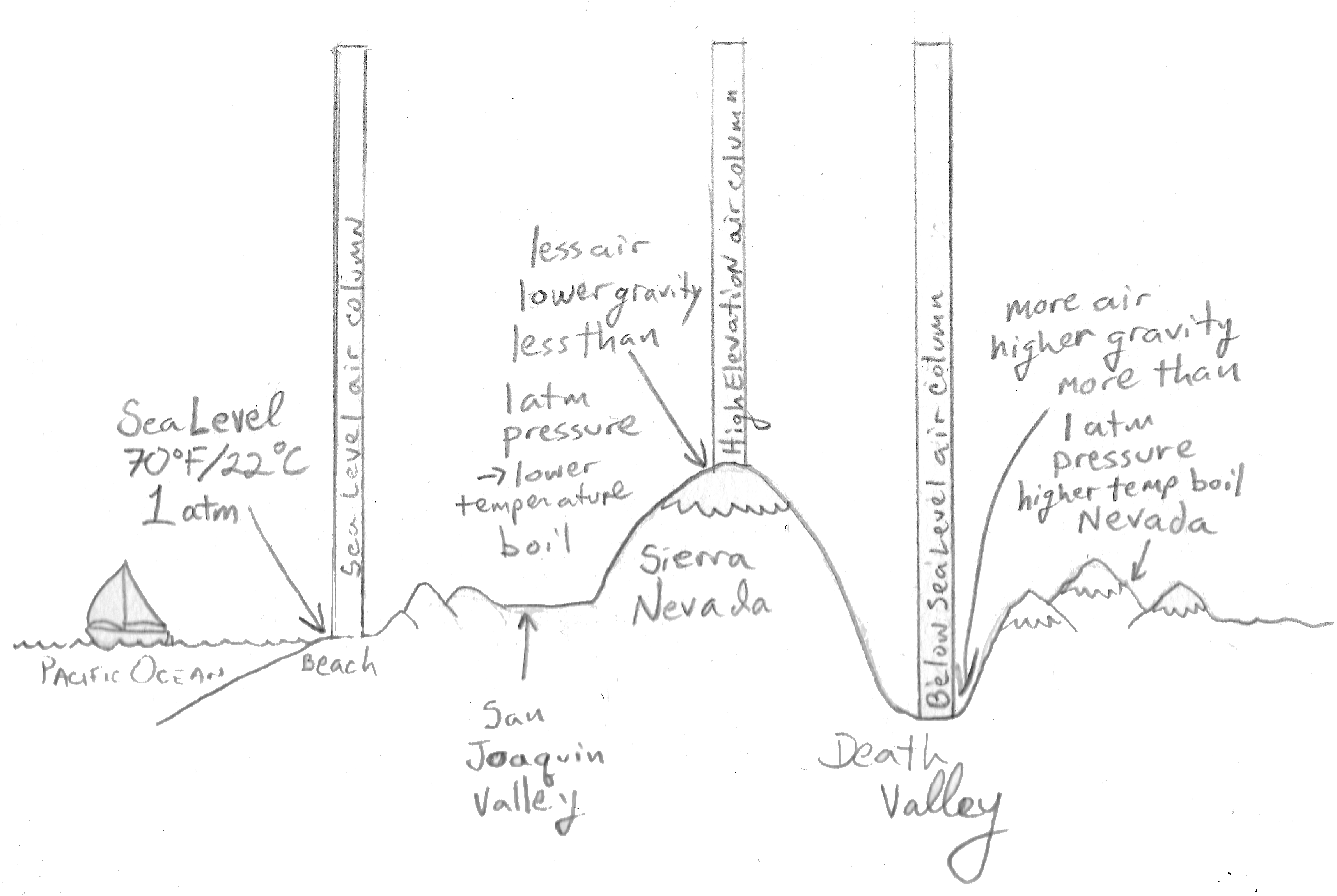
Why Elevation Changes Boiling Temperature
Let’s move our pot of water from Pismo Beach to the top of Mt. Whitney in the Sierra Nevada Mountains. Let’s keep the temperature at 68 ºF (20 ºC). Nothing changes as far as the pot of water is concerned. What does change is the air column on top of the pot. Because we are now at a much higher elevation, two things have happened:
- The air column has lost volume: all of the air that was originally between sea level and the top of the mountains is now missing. The result is that the air column now has a lot less mass.
- Gravity is lessened the further we get from the center of the Earth so everything weighs less at higher altitudes, including gas molecules.
Not only is there less mass in the air column, it weighs less too because of lower gravity. What this means is that the air column, which is a proxy for the atmosphere as a whole, now exerts less pressure on the water in the pot. Mt. Whitney is 14,505 ft or 4421 m high so atmospheric pressure is only 0.58 atm.
Now here’s where the concept of vapor pressure becomes really useful. Water will boil when the vapor pressure equals atmospheric pressure. If the atmospheric pressure at the top of Mt. Whitney is 0.58 atm, then water will boil when its own vapor pressure is equal to 0.58 atm. You only need to heat water to 85.5 ºC (186 ºF) for its vapor pressure to equal 0.58 atm.
Because atmospheric pressure is lower at higher elevations, water will boil at lower temperatures.
Now let’s move our pot of water some tens of miles to the east, to Death Valley. Death Valley is one of those places that is actually at a lower elevation compared to sea level, approximately 300 ft (91 m) lower. This means the column of air on top of the pot is some 300 ft (91 m) longer so it has gained some more mass. Not only that, but the force of gravity is greater because we are now a bit closer to the center of the Earth. The result is that the air column in Death Valley weighs more than the one at Pismo Beach at sea level, and therefore it exerts more than 1 atm of pressure on the water in the pot. At an elevation of 300 ft (91 m) below sea level, atmospheric pressure is about 1.05 atm.
So in Death Valley, the vapor pressure of water has to match the atmospheric pressure of 1.05 atm in order for water to boil; and that won’t happen until water reaches a temperature of about 102 ºC (216 ºF).
Because atmospheric pressure is higher at lower elevations, water will boil at higher temperatures.
Why Adding Salt Changes Boiling Temperature
Water boils at the temperature where its vapor pressure equals the pressure exerted by the local atmosphere. Now let’s add some salt to the water. Salt is a non-volatile solid, which is the same as saying that it has no vapor pressure. When you add a substance like salt into water, the added salt molecules now have the effect of diluting the number of water molecules per unit volume.
Since salt has no vapor pressure, the vapor pressure of the salt-plus-water solution is actually lower than that of plain water. If we are back at Pismo Beach, then we need the vapor pressure of any liquid to equal 1 atm in order for it to boil. Since the vapor pressure of salt water is less than plain water, that means that at 100 ºC, its vapor pressure is less than 1 atm. We need to heat the salt water to a higher temperature than 100 ºC in order for its vapor temperature to reach 1 atm.
Salt water boils at a higher temperature than plain water because its vapor pressure is lower.
How much of an effect does salt really have on the temperature of boiling? To answer this question, I dug back into my college physical chemistry texts and figured out how to calculate this. The equation I used is:
ΔTb = Kb · bsolute · i
where ΔTb = the change between the boiling point of a pure solvent vs. solution,
Kb = the ebullioscopic constant, which for water is 0.512 (°C·kg)/mol,
bsolute = the molality of the solution,
and i = a constant to account for non-ideal disassociation of the solute in the solution, which for salt is equal to 1.9
To set up the calculation, I noted or measured the following:
- 4 cups of water = 0.96 liters
- a tablespoon of salt weighs approximately 15 grams on my digital kitchen scale
- the molecular weight of salt is 58.4 grams/mole
- there are 0.257 moles of salt in a tablespoon.
- molality is measured in terms of moles per volume
- the molality of one tablespoon of salt in 4 cups of water is 0.257 moles/0.96 liters = 0.268
Having assembled all the pieces, the rest involved taking all the numbers and crunching them through the change-in-temperature equation. I plotted the results, which are shown in the graph below:
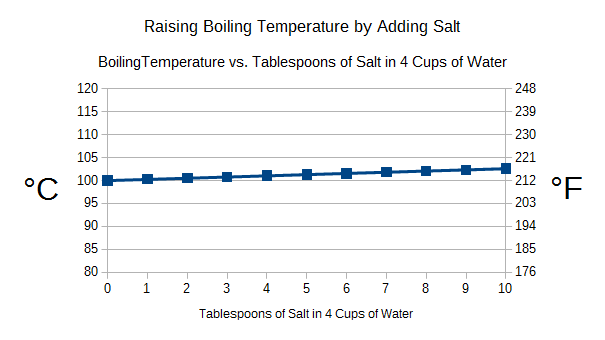
The results may surprise you. Even if you add 10 tablespoons of salt to four cups of water, you are only going to raise the boiling temperature by 2 °C (~4 °F). That’s less than a 2% increase in temperature. In a sound bite, that’s a trivial increase.
If we look at just adding one tablespoon, which is more than most people add for boiling eggs or making pasta, the boiling temperature is raised only by 0.3 °C (~0.5 °F). That’s less than the error of a typical kitchen digital food thermometer. Essentially, you can’t even measure the increase with the thermometers you have in your house.
The bottom line is that you are wasting your salt. The amount that the boiling temperature is raised is so small you can’t even measure it accurately.
PARTS II and III
Next week, on December 3, we will cover the biochemistry of what’s inside an egg. The week after, on December 10, we will go into the what happens inside the egg when you cook it inside the shell and finally address that all important question: how to make the perfect hard-boiled egg.
SOURCES
- https://van.physics.illinois.edu/qa/listing.php?id=1457
- P. W. Atkins, Physical Chemistry, 4th Ed., W. H. Freeman & Co, NY, 1994, p. 222-225 & p. C17
- P W Atkins and J. de Paula, Physical Chemistry, 8th Ed., W. H. Freeman & Co, NY, 2006, p. 151-153
- http://vaxasoftware.com/doc_eduen/qui/tcriosebu.pdf
- https://www.chem.purdue.edu/gchelp/liquids/vpress.html – extremely good site on vapor pressure
- https://www.chem.purdue.edu/gchelp/liquids/vpacetn.html
- http://www.endmemo.com/chem/vaporpressurewater.php
- https://www.mide.com/pages/air-pressure-at-altitude-calculator
The banner image of boiling eggs for today’s post is by “ProjectManhattan” (Own work) [CC BY-SA 3.0], via Wikimedia Commons.
The Pulp-O-Mizer image was made with the Pulp-O-Mizer, a free DIY graphics tool by artist and writer Bradley W. Schenck. No, this is not an ad and I didn’t get paid to write this. I just like the Pulp-O-Mizer a lot and think it’s cool that you can make your own Pulp-O-Mizer images for free non-commercial use on your website or blog.

7 thoughts on “The Food Science of Hard-Boiled Eggs – Part I: Boiling Water”
Your illustration is using T=70F, not 68. It doesn’t really matter, but I think it should be consistent with the text.
The evaporating water at the surface doesn’t really do anything per se except that you can measure it’s pressure. It’s the fact that at boiling temperature the nucleated bubbles can actually lift the water around them as well that causes the bubbling action of boiling, which means that the temperature will be marginally higher than the temperature at which the vapor pressure is equal to one atmosphere.
Yeah, I’m picky.
I made the figure before I wrote the text. It takes some non-trivial time to draw, scan, and clean-up the figures. I knew the figure and the text were out of sync, but it wasn’t worth it to me to spend an hour-plus fixing the figure for something I’m not getting paid to do. Sloth is my favorite of the seven-deadly sins. Yeah, I’m lazy.