The Food Science of Hard-Boiled Eggs – Part II
This is the second of three parts on the eggcellent topic of hard-boiled eggs.
To state the obvious, this discussion is about the unfertilized eggs of chickens, ducks, and geese.
THE BIOCHEMISTRY OF EGGS
The problem with making the perfect hard-boiled egg is that everyone has their own favorite recipe or gimmick that works for them; however, few people understand why their own method works or why someone else’s doesn’t. To understand how to properly hard boil an egg, we first need to discuss what’s inside that shell. The biochemistry of an egg matters when talking about the science of cooking it.
If you read and understood PART I last week on the physical chemistry of boiling water, and you digest today’s article on what’s inside an egg, then you’ll have no problem next week when we describe how cooking with science is a far better way to make that perfect hard-boiled egg than relying on an internet full of everyone’s favorite hard-boiled gimmicks.
The Physical Structure of an Egg
The Shell
- The outside of a poultry egg is that hard layer we all know and curse while cracking it open: the shell.
- The shell is mostly calcium carbonate with the approximate structure of the mineral aragonite.
- The shell is actually riddled with itty bitty pores to permit gas exchange. Without pores, the chick that grows inside a fertilized egg would suffocate.
The Inner and Outer Membranes
- Immediately inside of the shell are two flexible-but-strong membranes. These containerize the sticky viscous contents inside the egg shell just like a plastic food-storage bag containerizes leftovers in the freezer or fridge.
- One of the main components of the membranes is the long-stringy protein keratin. Keratin is the same stuff that makes fingernails, hair, and skin so tough.
- When eggs get laid, they are warm. As they cool down, the liquid contents contract within the rigid shell which causes a gap to form between the inner and outer membrane at the fat end of the egg. This gap is called the air cell.
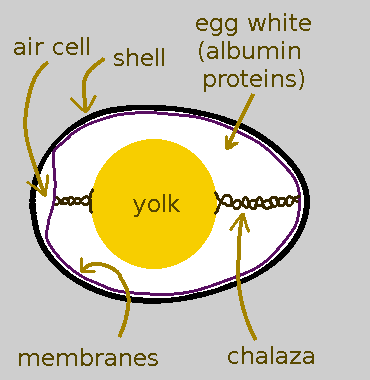
The Egg White
- Moving inward, just inside the membranes is the egg white.
- Egg white is made up of water and albumin proteins. Albumin is sometimes spelled as albumen; which version is correct depends on who you ask.
- The purpose of the egg white is to protect the yolk.
- If the yolk were fertilized, the egg white would also serve as a source of nutrients for the growing embryo.
- This will important later: some of the proteins in egg white have sulfur in them.
The Yolk
- The unfertilized yolk is made up of all sorts of stuff: more proteins, water, some fats, the fatty compound lecithin, vitamins, minerals like iron, etc.
- If the yolk had been fertilized, it would have become site of a growing embryo that would eventually have been born as a chick.
- There is a thin sack around the yolk called the vitelline membrane (not shown in the egg diagram) to keep it separate from the egg white.
- Some of the protein in the yolk also has some sulfur in them; however the amount is small, only about a tenth of the sulfur in egg white.
Chalazae
- The yolk is held in the middle of the egg by two white strands called chalazae (singular: chalaza).
- If you crack open an egg, you can gauge how fresh it is by how white the chalazae are. White, prominent chalazae mean your egg is really fresh.
- Chalazae are NOT any part of an embryo, contrary to popular folk lore.
- Chalazae are edible. When cooked, they can’t be distinguished from cooked egg whites.
- Chalazae are more dense than egg white so you’ll want remove them if you are making meringue or custard. You won’t have a uniform texture otherwise.
Egg Gas and the Air Cell
The air cell is that gap between the inner and outer membranes just inside the shell of the egg, formed by the contraction of the cooling egg contents after the egg is laid. Though usually at the fat end of the egg, the air cell can actually form elsewhere, like between the membranes at the pointy end of the egg. The air cell can also break up to form many little air gaps instead. Storing your eggs upright in a carton will help keep the air cell in place.
- The location of the air cell at the fat end of the shell is why the bottom of a hard-boiled egg is flattened once you peel it.

The Effect of the Air Cell and Egg Gas on the Membranes is Important!
The physics of what happens to the air cell and the membranes as an egg ages is important to the science of cooking and peeling of hard-boiled eggs.
- The gas that initially occupies the air cell is mostly carbon dioxide and a little water vapor, both of which were sucked out of the egg white as the air cell formed.
- As time passes and the egg ages, some of the water and gasses from the air cell spreads between the two membranes, helping to separate them.
- Because the shell is porous and permits gas exchange, most of the carbon dioxide and water vapor leave the egg to be replaced by plain old atmospheric air. There are two mechanism at work here that make this happen:
- First, because the air cell formed through contraction of the liquids in the egg, its pressure is initially lower than the atmosphere, so air is sucked in through the porous shell.
- Second, nature dislikes it when gasses on either side of a permeable membrane – in this case, the porous shell – are not in equilibrium. Water and carbon dioxide inside the air cell will leave the air cell and gasses in the atmosphere like nitrogen and oxygen will push their way in until the composition of gas in the air cell is the same as the atmosphere outside the shell.
- As the egg ages, carbon dioxide continues to diffuse out of the egg white and eventually out of the egg. This has the effect of shifting the pH of the egg to more alkaline values.
- The initial pH of an egg is about 7.6, which is only a little bit alkaline.
- As carbon dioxide leaves the egg and makes its contribution to global warming, the pH of the egg can shift to almost 10 a few days after it was laid.
- When the pH of an egg is ~8.7 or higher, the egg white no longer wants to cling to the inner surface of the inner membrane.
You can probably already guess that all this stuff about the air gap and the membranes has a lot to do with how easy or hard it will be to peel a hard-boiled egg.
Come Back Next Week for Part III
We’ve laid the groundwork here and in Part I for you to grok the science behind the perfect hard-boiled eggs. Next week on December 10, we’ll wrap up the biochemistry, look at what happens to egg proteins when you when you cook them, and explore better egg-shell peeling through physics and chemistry. It’s no eggsaggeration to say that’s it’ll be great! So come back next week for the conclusion of the food science of hard-boiled eggs, same bat time, same bat channel!
SOURCES
- On Food and Cooking: The Science and Lore of the Kitchen, Harold McGee, 2004 Scribner NY ISBN:978-0684800011
- http://www.incredibleegg.org/eggcyclopedia/a/albumen/
- http://www.incredibleegg.org/eggcyclopedia/y/yyolk
- http://www.incredibleegg.org/eggcyclopedia/c/chalazae
- http://www.incredibleegg.org/eggcyclopedia/c/composition/
- http://www.exploratorium.edu/cooking/eggs/eggscience.html
- https://www.exploratorium.edu/cooking/eggs/eggcomposition.html
- http://kitchenpantryscientist.com/the-science-of-hard-boiled-eggs/
- http://chestofbooks.com/food/science/Experimental-Cookery/Properties-Of-Egg-Proteins-Part-1.html
- http://chestofbooks.com/food/science/Experimental-Cookery/Properties-Of-Egg-Proteins-Part-2.html
- http://chestofbooks.com/food/science/Experimental-Cookery/Properties-Of-Egg-Proteins-Part-3.html
Today’s banner graphic is a 1911 political cartoon from the presidency of William Howard Taft, which was a time of rapid inflation, government deficit, the first formal annual federal budget, federal tariffs on multiple commodities and the creation of the first income tax.

2 thoughts on “The Food Science of Hard-Boiled Eggs – Part II”